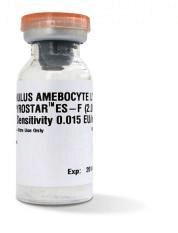Kit of 4 PYROSTAR™ ES-F (2ML)
with control standard endotoxin (CSE)
- Multi test vial (4 x 2 ml + 1 CSE)
EXPECTED USE
SUMMARY AND GENERAL INFORMATION
The US The Food and Drug Administration (FDA) issued a guideline in 1987 to inform manufacturers of human drugs and biologicals, veterinary drugs, and medical devices of the procedures that the FDA considered necessary to validate the use of LAL as a final product for endotoxin tests. The FDA guideline was combined with the USP bacterial endotoxin test in 2000, making the USP method the standard method for manufacturers in the US. The 1987 FDA guideline was withdrawn in 2011.
HISTORY AND BIOLOGICAL PRINCIPLE
The US The Food and Drug Administration (FDA) issued a guideline in 1987 to inform manufacturers of human drugs and biologicals, veterinary drugs, and medical devices of the procedures that the FDA considered necessary to validate the use of LAL as a final product for endotoxin tests. The FDA guideline was combined with the USP bacterial endotoxin test in 2000, making the USP method the standard method for manufacturers in the US. The 1987 FDA guideline was withdrawn in 2011.
GENERAL WARNINGS AND PRECAUTIONS

Obtain updated infomation on inveronmenteal monitoring and technical advice in our E-BOOKS.
Obtain updated infomation on inveronmenteal monitoring and technical advice in our E-BOOKS.

Contact us
?? Argentina:
Buenos Aires. Argentina
Sargento Cabral 2431
Vicente López
CP 1605
Telf: +54 9 11 3027-3162
?? Paraguay Affiliate:
Asunción, Paraguay
Misiones 593 e/ Perú
CP 1102
Telf : 0972-104600
?? EEUU Affiliate:
Florida. EEUU
1101 Brickell Avenue South, Tower 8th Floor, Miami,
FL 33131
Telf: (305) 381- 1855
Email adress:
ventas@mikrobiol.com